Merck Life Sciences banks on RPA to streamline regulatory compliance
CIO
JUNE 5, 2023
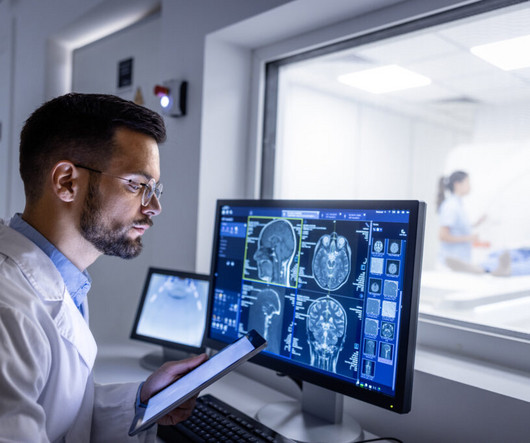
The pharmaceutical industry is a highly regulated one, especially for multinationals doing business across the globe. The firms’ trade compliance teams must not only engage with all these processes but ensure they are aligned with ever-increasing regulations, which can differ notably from country to country.















































Let's personalize your content